Request a quote.

Electrocoagulation Technology for Superior Wastewater Treatment
Electrocoagulation is based on principles similar to those of the widely used chemical coagulation, making the two technologies easily comparable. Chemical coagulation itself is a highly versatile process, removing a major part of the commonly occurring contaminants in wastewater. The process is usually carried out in large basins. The term ‘coagulation’ refers to the ability of multiply charged metal ions to form hydroxocomplexes attracting dissolved contaminants to adsorb onto the surface of the complex in a significantly sub-stoichiometric manner and then adding a flocculation polymer to form larger complexes.
In chemical coagulation, the coagulation is achieved by means of the addition of a strongly acidic and corrosive aqueous solution of e.g. ferric or aluminium chloride followed by a neutralization using sodium hydroxide. In electrocoagulation, the coagulation is achieved by means of letting a direct current dissolve a sacrificing electrode made of metal over time. In this process hydroxide ion is formed as the counter-ion, thus omitting the need for neutralization and not adding any chloride ion to the solution as is usually the case in chemical coagulation. Hydrogen gas is also formed in the electrochemical process.
By avoiding harsh chemicals and producing fewer byproducts, electrocoagulation technology aligns with the growing demand for sustainable wastewater treatment methods. It is an ideal choice for industries seeking efficient, environmentally friendly solutions to meet strict environmental regulations while maintaining cost-effectiveness.
The Principles of Coagulation
The main mechanism at work, regardless if EC or traditional flocculation with chemicals is used, is coagulation. It is without doubt one of the most important physio-chemical reactions used in water treatment. Simplified, the various pollutants in water, suchas ions (heavy metals) and colloids (organic andinorganic), are basically held in solution by repulsive electrical charges. The addition of ions with opposite charges destabilizes the colloids, allowing them to coagulate. Such coagulation occurs regardless of if the coagulation is achieved by means of adding achemical coagulant or by utilisation of EC technology.

Benefits of Electrocoagulation Compared to Chemical Coagulation
In traditional chemical coagulation, commonly used agents include aluminum sulfate, polyaluminum chloride, and ferric chloride—substances that have been staples in wastewater treatment for decades.
Electrocoagulation, however, operates through a fundamentally different mechanism. As previously mentioned, it generates coagulants in situ through the electrolytic oxidation of a suitable anode material. During this process, charged ionic species in the wastewater react with oppositely charged ions or with metallic hydroxide flocs that form directly in the effluent. The effectiveness of electrocoagulation is further enhanced by several concurrent side reactions—most notably, the production of microhydrogen bubbles on the cathodes. These tiny bubbles offer a large surface area for particles to adhere to, significantly boosting pollutant removal efficiency. Together, these reactions contribute to the distinct advantages of electrocoagulation, which can remove a wider range of contaminants than many conventional treatment methods.
Traditional chemical coagulation methods, where external chemicals are added to destabilize suspended particles and precipitate pollutants, have well-known limitations. One of the most significant drawbacks is the generation of large volumes of sludge with a high water content. This type of sludge is difficult to filter and dewater, making its treatment and disposal both challenging and costly—often forming one of the largest expenses in wastewater treatment operations, particularly when chemical coagulants are involved.
Additionally, chemical coagulation typically increases the total dissolved solids (TDS) content in the treated water, which can hinder water reuse and recycling in industrial applications.
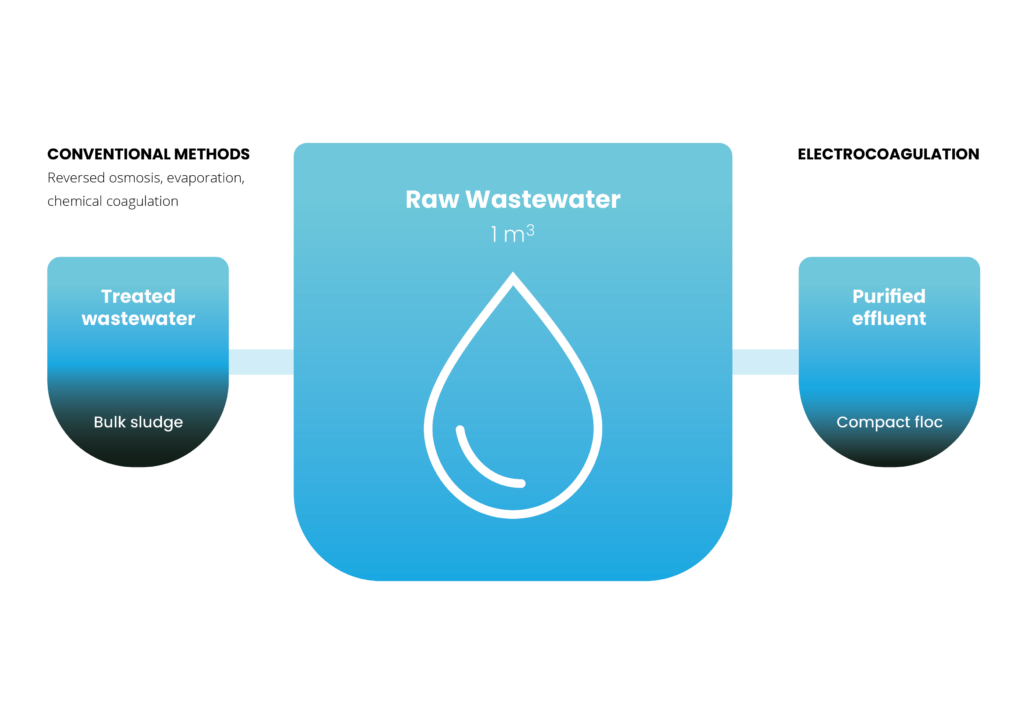
Electrocoagulation offers numerous advantages over conventional chemical coagulation, including:
No need to handle hazardous chemical solutions or maintain large chemical storage tanks, improving safety and saving space.
Compact storage requirements for replacement electrodes or reactors, making it well-suited for remote locations and emergency response scenarios.
Production of dense, high-solids floc that is easier to dewater, reducing handling and disposal costs.
A higher yield of reusable, clean water without chloride contamination, facilitating on-site water recirculation.
Sludge generation is reduced by 5 to 10 times compared to chemical coagulation, leading to up to 50% lower operational costs.
These benefits position electrocoagulation as an efficient, cost-effective, and environmentally sustainable alternative for modern wastewater treatment systems.

The Benefits of AxoPur
AxoPur is Mellifiq’s engineering adaption of the electrocoagulation technology. The AxoPur reactor ensures all wastewater to actually pass through the reactor and be subjected to an electric field and to a homogenous treatment. Additional cleaning mechanisms integral to AxoPur include effects by the electric field and the formation of hydrogen gas in the electrochemical reaction. Overall the result is an extraordinarily efficient broad-spectrum wastewater treatment – a one-stage purification.
Many electrocoagulation systems operate batch-wise and require significant treatment times, limiting their capacity. Contrary to this, the reaction in the AxoPur reactor is instant and the system designed for continuous operation, although intermittent operation is always an option, should the flow not be there. Since the reaction is instantaneous, the system has a compact footprint. It also means the water will keep its temperature throughout the process.
AxoPur: Benefits compared to general electrocoagulation
AxoPur enhances wastewater treatment by ensuring all water passes through the reactor for higher purification efficiency. It removes a wider range of contaminants, including oil, fat, microbes, and PFAS, due to additional mechanisms. The process allows for quick and simple adjustments through current changes, and it retains water temperature, enabling heat recovery when purified water is recirculated.



